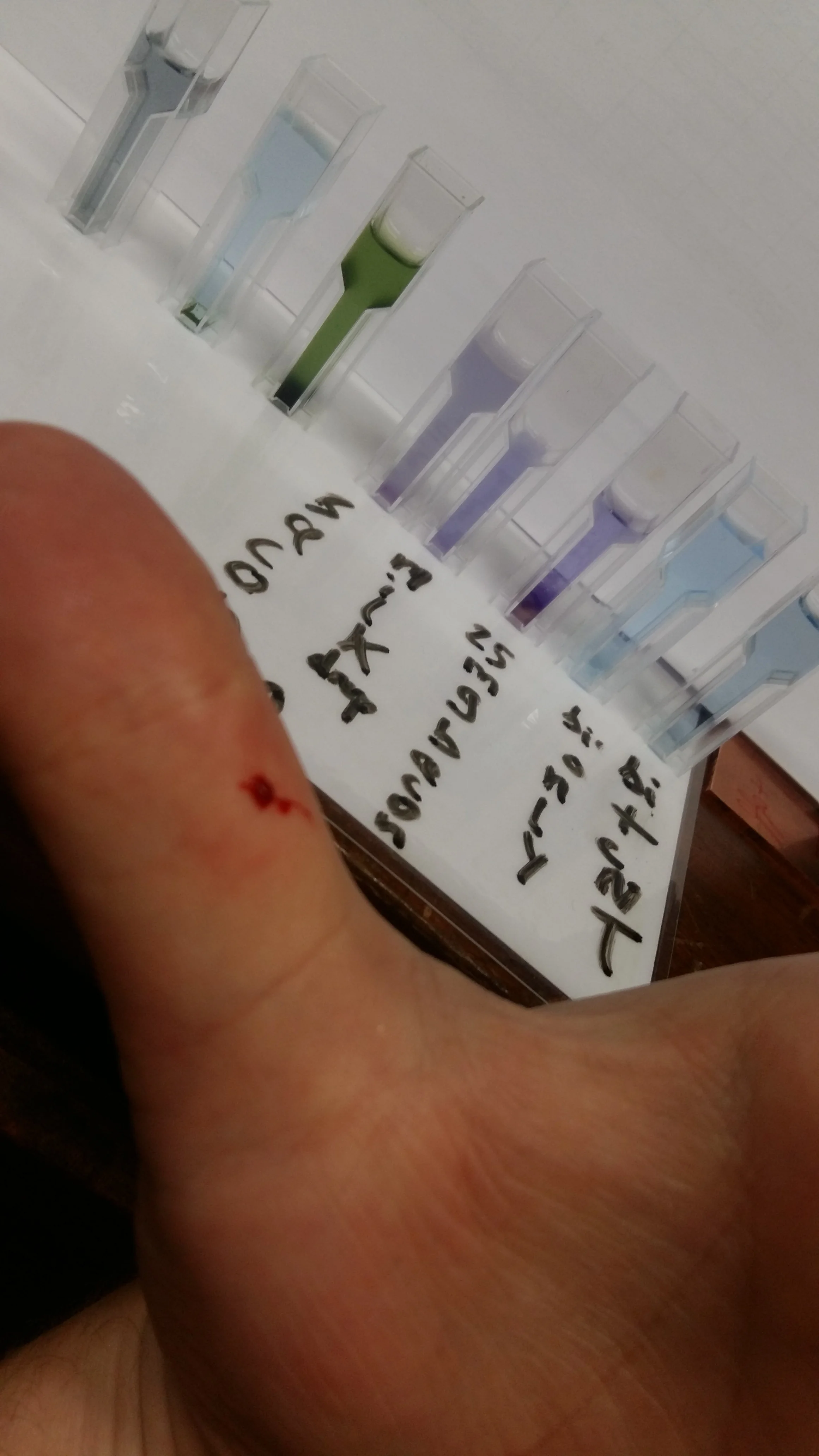
Biuret Reagent tests for the presence of protein. Will whole human blood, dropped in the solution, indicate positive?
No, I didn’t heroically prick my finger to see if it would turn Biuret reagent purple, this was a fortuitously timed papercut!
But it raised a fun question: would a drop of whole human blood give a positive Biuret test for protein?
Whole blood in Biuret reagent. Plasma proteins react instantly, but haemoglobin’s strong red pigment does its best to hide the evidence.
The Biuret Test:
Biuret reagent is a straightforward protein test. It works because copper(II) ions form a violet-coloured complex when they coordinate with peptide bonds, provided the chain is at least three amino acids long. Since proteins are long chains of amino acids, the logic is: no protein, no colour shift.
It’s a staple of school science labs, where it reliably turns egg-whites or milk purple coloured. But whole blood isn’t quite milk. And aren’t the proteins wrapped up securely inside cell membranes?
What’s in a Drop of blood?
Surprisingly, most of the protein in blood isn’t locked inside cells - it’s floating in the plasma. Blood plasma (the pale yellow fluid left after you remove the cells) is absolutely loaded with proteins, including:
• Albumin (~60% of plasma protein): maintains osmotic pressure and acts like a taxi for hormones, drugs, and fatty acids.
• Globulins (~35%): a diverse group including antibodies (immunoglobulins), transport proteins, and clotting factors.
• Fibrinogen (~4%): key for clot formation - without it, even a paper cut could be a disaster.
These proteins are fully dissolved in plasma and chemically accessible, making them prime candidates for detection by the Biuret test.
On the other hand, red blood cells (RBCs) do contain a ton of protein - mainly haemoglobin - but it’s sequestered inside the cell membranes. Unless the cells are lysed (ruptured), the Biuret reagent won’t “see” these internal proteins.
What Happened..
In this case, a couple of small blood droplets from a papercut were sprinkled into a vial of Biuret reagent.
The result?
A dark purple-red colour developed almost immediately. It wasn’t the clean violet seen with egg white or milk, but the shift was visible to the eye, and easy to capture on a smartphone. The red of the haemoglobin didn’t fully obscure the colour change, though it likely did dull the clarity of the result.
So yes, whole blood does yield a positive result.
But…
In my tiny papercut droplet, the total protein content was too low to give a substantial, unambiguous violet shift. And haemoglobin’s intense red pigment tends to dominate. It absorbs across the visible spectrum and masks the more subtle violet tone of the Biuret-copper complex.
The result is a kind of muddy, reddish-purple that tells you something happened, but not how much. That’s a problem if you want to quantify the changes.
To measure protein concentration properly, you’d need to switch from your eyes to a spectrometer, ideally set around 540 nm, and compare your result against a calibration curve made from known protein standards. That way, even small concentration changes can be quantified.
A Better Way?
Biuret reagent is simple, but not particularly sensitive. For low-concentration samples, there are better tools:
Bradford assay (based on dye binding)
Lowry method (includes a redox reaction)
Bicinchoninic acid (BCA) assay (a more sensitive variation on Biuret chemistry)
These are standard in biochemistry labs because they respond more clearly to small amounts of protein.
Thesis Tax!
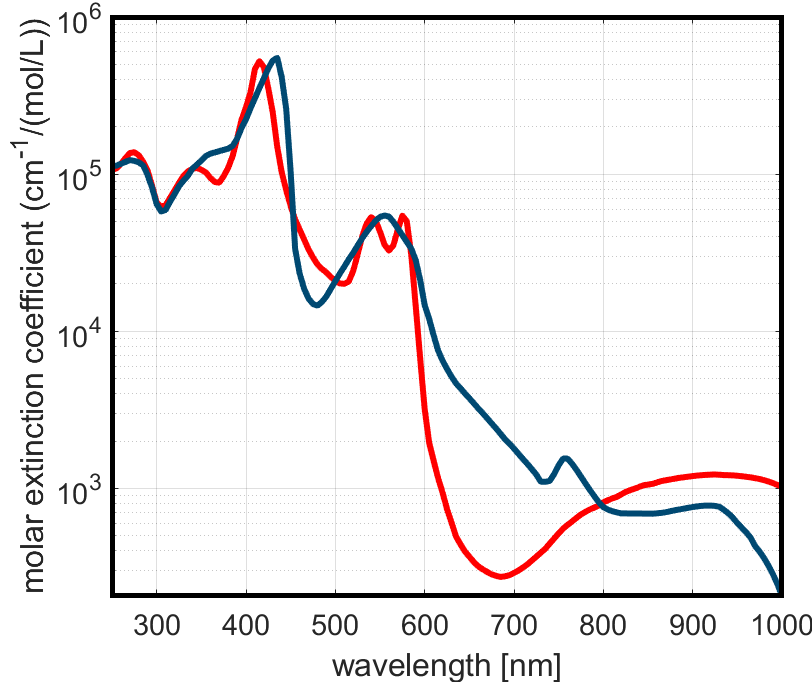
Absorption spectra of oxy- (red) and deoxy-haemoglobin (blue), adapted from Prahl et al. (1993). Strong peaks in the green-yellow and blue regions explain why violet shifts from the Biuret reagent can be hard to see, as haemoglobin absorbs most of the visible spectrum, especially where you’d want your purple to shine through.