Can a free electron absorb a photon?
Short answer is no, it can't happen. There is no accepted mechanism for the strictly free electron to absorb a quanta of electromagnetic radiation.
How would the electron "hold on" to the photon? (Increase the electron energy perhaps?)
As far as we know, the electron has no substructure...
We say it can't happen because it violates simultaneous energy and momentum conservation.
Here's one (arguably unsatisfying) reason why:
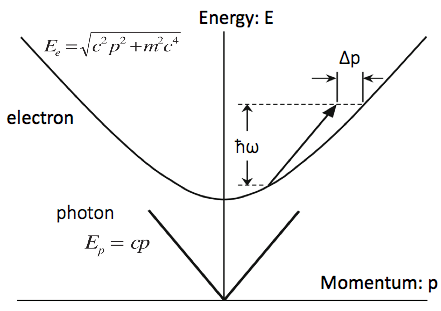
This is a plot of the free electron and photon dispersion relations in free space, or how their energies look like at any given momentum. (The rules are set: if the particle has an energy, it has a specific momentum, and vice versa). Note that, because of the way the electrons dispersion relation is curvy shaped, there's just no way to take a given photon line and successfully slap it on the electrons curve. It always comes up short.
So the electron can't be at some energy/momentum, then fully absorb a photon and jump up to some higher energy/momentum point on the curve.
In fact, the momentum amount it comes up short by, delta p, equals hbar omega over the electron velocity.
The main consequence of all this is that a 3rd body is required nearby to provide momentum - this is where the bound electron can absorb and emit photons all day long, jumping up and down electron energy levels.
If no 3rd body is present, free electron sees the photon, get's jiggled as it passes, but then returns to exactly what it was doing after the photon is gone. No NET absorption happens, as far as we know.
More on quora.